The goal of NIH BRAIN Initiative Public-Private Partnership program is to facilitate partnerships between clinical investigators and manufacturers of latest-generation stimulating and/or recording devices that are designated by the U.S. Food and Drug Administration (FDA) as Class III (invasive, posing significant risk of harm), to conduct clinical research in the central nervous system (CNS). As part of the BRAIN Initiative, NIH is interested in reducing barriers to negotiating such partnerships and also ensuring that new clinical studies leverage manufacturers' existing data demonstrating safety and utility of these devices, data that are very costly to obtain and pose a substantial barrier to research progress.
To advance these goals, NIH has signed Memoranda of Understanding (MOUs) with device manufacturers that serve as the basis for this program. Each participating company has provided information on devices and support they are willing to provide to researchers. This information serves as a guide for investigators wishing to pursue specific agreements with manufacturers for submission of research proposals to the NIH. To streamline legal and administrative process for partnerships between manufacturers and academic research institutions, NIH has created template Collaborative Research Agreements (CRA) and Confidential Disclosure Agreements (CDA) that will serve as a common starting point for these partnerships. The templates are based on drafts that received substantial input from clinical researchers, representatives from government agencies, including the U.S. Food and Drug Administration, representatives from the medical device industry, from institutional tech-transfer and contracts offices, and from the general public.
For this program, NIH has entered into agreements with a number of manufacturers to make available next generation devices that can stimulate and/or record from the central nervous system and have sufficient data to enable new Non-Significant Risk (NSR) or Investigational Device Exemption (IDE) without the need for significant additional testing. See the links below for current Notice of Funding Opportunity (NOFOs) and notices, funded awards, resources, and more.
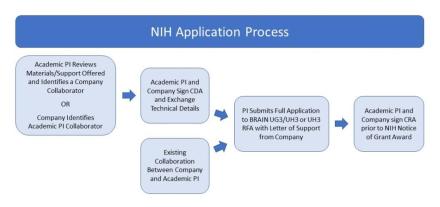
MOU, CDA, and CRA refer to Template Research Agreement Documents. UG3 and UH3 refer to funding activity codes associated with the funding opportunities.
Collaborative Research Agreement Template
This is a template document(pdf, 482 KB) to be used for agreements between device manufacturers and academic research institutions to form partnerships for submission of grant applications to the NIH for clinical research. The goal of this document is to provide standardized terms covering essential components of such agreements (e.g., intellectual property, data and publications, reporting requirements, etc.). In some cases, the template language may be used "as is" whereas in other cases it may be a starting point for discussions. For a Word document version of the template agreement, please contact Dr. Megan Frankowski. Drafts of this and other documents were the focus of specific discussions at a June 2015 BRAIN Initiative workshop titled, “Industry Partnerships to Facilitate Early Access Neuromodulation and Recording Devices for Human Clinical Studies.” Information on the workshop and links to videocast proceedings are available in the June 2015 Workshop materials.
Confidential Disclosure Agreement Template
This template document(pdf, 367 KB) can be signed by academic researchers to allow confidential discussions of proprietary details with a given company regarding the capabilities of a device for the conduct of clinical research. For a Word document version of the template agreement, please contact Dr. Megan Frankowski. Drafts of this and other documents were the focus of specific discussions at a June 2015 BRAIN Initiative workshop titled, “Industry Partnerships to Facilitate Early Access Neuromodulation and Recording Devices for Human Clinical Studies.” Information on the workshop and videocast proceedings are available in the June 2015 Workshop materials.
Contact
Megan Frankowski, Ph.D.
National Institute of Neurological Disorders and Stroke (NINDS)
megan.frankowski@nih.gov
